Peptide-nucleic acids for CFTR correction
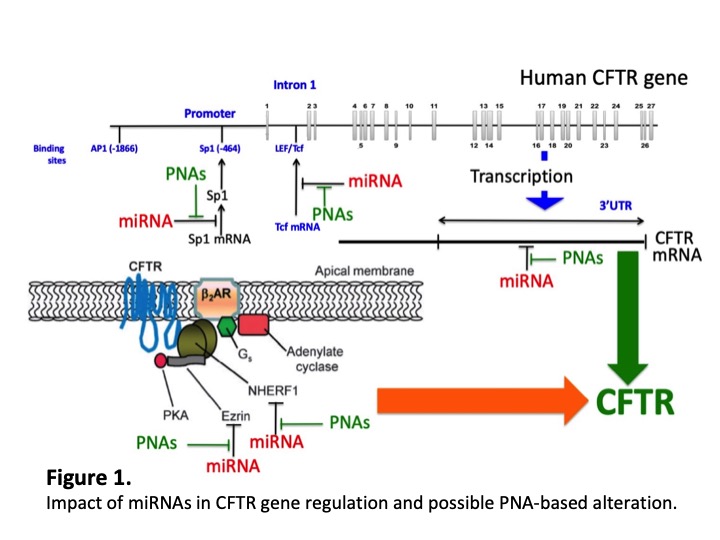
MicroRNAs as key post-transcriptional regulators of gene expression. MicroRNAs (miRNAs) are a family of small (19 to 25 nucleotides in length) noncoding RNAs that regulate gene expression by sequence-selective targeting of mRNAs, leading to a translational repression or mRNA degradation, depending on the degree of complementarity between miRNAs and the target sequences. While miRNAs usually interact with the 3’UTR region of mRNAs, functional binding to coding sequences and 5’UTR have also been described. Since a single miRNA can target several mRNAs and a single mRNA may contain several signals for miRNA recognition, it is calculated that at least 10-40% of human mRNAs are targets of microRNAs. In general, a low expression of a given miRNA is expected to be potentially associated with accumulation of targets mRNAs; conversely, a high expression of miRNAs is expected to be the cause of a low expression of the target mRNAs.
MicroRNA Therapeutics for Cystic Fibrosis. Since the involvement of microRNAs in human pathologies is a firmly established fact, the pharmacological modulation of their activity appears to be a very interesting approach in the development of new types of drugs (miRNA therapeutics). One of the most important research areas is the possible development of miRNA therapeutics for genetic diseases. For instance, miRNAs are involved in Cystic Fibrosis (CF), a hereditary disease caused by alteration of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) gene. In fact, miRNAs expression has been recently explored by different groups in CF primary bronchial epithelial cells in vitro or from bronchial brushings ex vivo. Expression of miR-145-5p and miR-494-3p was found anti-regulated with that of CFTR. Genome-wide expression of miRNAs in primary non-CF bronchial epithelial cells revealed the expression of miR-138 as a down-regulator of SIN3A, a transcriptional repressor of CFTR. A second study of miRNA profiling showed a high expression of miR-494-3p and miR-509-3p in the CF cells and a direct interaction with CFTR transcript. These in vitro findings were confirmed and extended in ex vivo analyses, which evidenced an increased expression of miR-494, miR-223 and miR-145 in CF brushings of airway cells. Altogether, different miRNAs which have been found increased in CF primary bronchial epithelial cells can reduce CFTR expression, either by direct (miR-145-5p, miR-223-3p, miR-494-3p, miR-509-3p, miR-101-3p) or indirect (miR-138-5p) targeting.
Peptide Nucleic Acids and microRNA therapeutics. With respect to miRNA therapeutics, peptide nucleic acid (PNA)-based molecules are appealing. In PNAs the pseudo-peptide backbone is composed of N-(2-aminoethyl)glycine units. PNAs are resistant to both nucleases and proteases and, more importantly, hybridize with high affinity to complementary sequences of single-stranded RNA and DNA, forming Watson-Crick double helices. For these reasons, PNAs were found to be excellent candidates for antisense and antigene therapies. The major limit in the use of PNA for alteration of gene expression is the low uptake by eukaryotic cells. In order to solve this drawback, several approaches have been considered, including the delivery of PNA analogues with liposomes and microspheres. One of the possible strategies is to link to PNAs to polyarginine (R) tails, based on the observation that this cell-membrane penetrating oligopeptides are able to facilitate uptake of conjugated molecules. Peptide-PNA conjugates have been shown to be efficiently incorporated in cells without the need of transfecting agents. Recently, PNAs have been shown to be able of altering biological functions of microRNAs, both in vitro and in vivo. With respect to PNA-based targeting of miRNAs involved in CFTR regulation, we have recently reported that a PNA directed against miR-145-5p inhibits its biological functions and up-regulates CFTR expression.
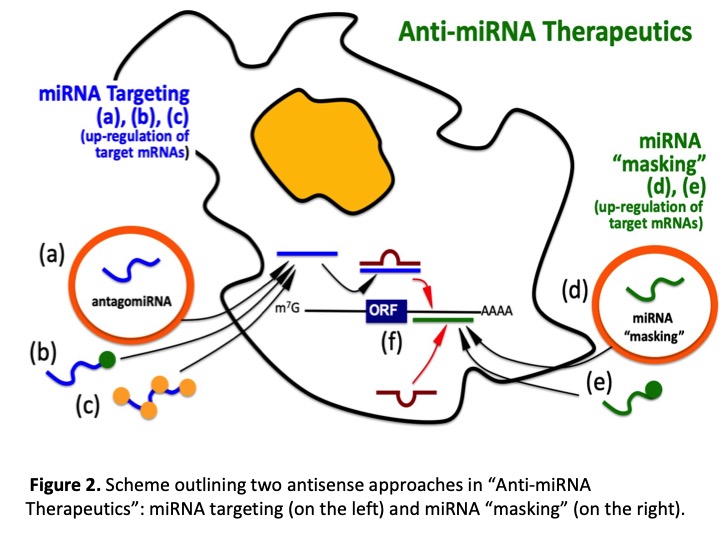
Hypothesis and objectives. (a) Characterization of key miRNAs regulating CFTR and CFTR modulators; (b) identification of mRNA targets of CF-associated miRNAs (including transcription factors controlling CFTR); (c) studies on changes of gene expression in CF (including CFTR activation) using antagomiRNA peptide nucleic acids (PNAs); (d) combined treatments using CFTR correctors and potentiators; (e) development of miRNA-masking PNAs; (f) characterization of miRNAs differentially present in body fluids of CF patients.
Essential methods. Next-Generation sequencing (NGS), RT-qPCR and digital RT-ddPCR, Western blotting.
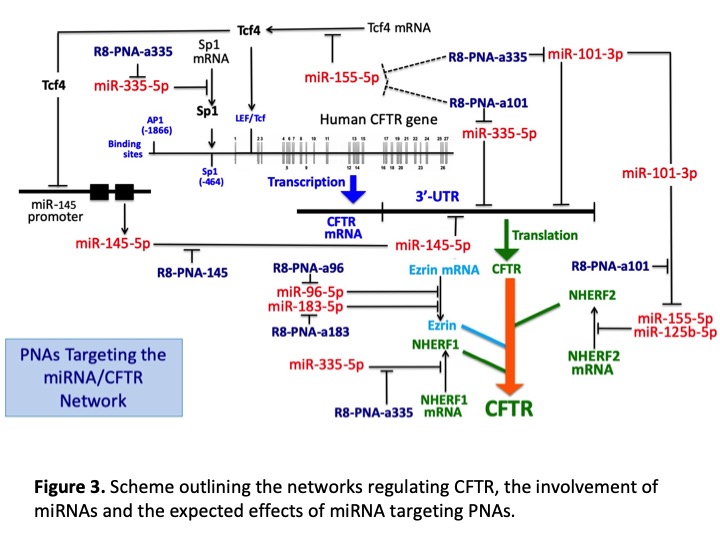
Results. PNAs against miR-145-5p, miR-494-3p, miR-101-3p (all targeting CFTR mRNA) induced increase of CFTR in Calu-3 cells. An increase of CFTR was obtained also with a PNA against miR-335-5p, targeting the CFTR modulator NHERF1 and by PNAs against miR-96-5p and miR-183-5p (both targeting ezrin mRNA). Specific and efficient PNA masking the miR-145-5p binding sites was demonstrated to increase CFTR. These PNA-based CFTR modifiers were studied in combination with CFTR potentiators and correctors. For example, maximum levels of CFTR increase were achieved in CFBE-41o-WT and CFBE-F508 by combining the miR145-maskingPNA with VX809 and VX770. In addition, the miR145-maskingPNA/VX809/VX770 combination was the most effective in stimulating CFTR-mediated chloride efflux in the CFBE41o- F508del CFTR YFP cell line. We have studied by NGS the global effects on the miRNome of treatments with PNA-a101, PNA-a335 and PNA-a145 demonstrating selective effects of the employed PNAs. We have concluded the characterization of novel delivery systems for PNAs and pre-miRNAs based on (a) porous silicon nanoparticles and (b) a macrocyclic multivalent tetraargininocalix[4]arene. Heterogeneity of miRNA profile was found in plasma samples from CF patients, suggesting personalized strategy for miRNA therapy.
Conclusions. The major output of CF-miRNA-THER is the identification of miRNA targeting in translational medicine, with the objective of modifying gene expression of cystic fibrosis cells, and, in particular, of increasing stability/expression of the CFTR protein. This therapeutic goal is very important for the treatment of cystic fibrosis.
Contacts:
Roberto Gambari (email: gam@unife.it) and Alessia Finotti (email: alessia.finotti@unife.it)
References
Tamanini A, Fabbri E, Jakova T, Gasparello J, Manicardi A, Corradini R, Finotti A, Borgatti M, Lampronti I, Munari S, Dechecchi MC, Cabrini G, Gambari R. A Peptide-Nucleic Acid Targeting miR-335-5p Enhances Expression of Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Gene with the Possible Involvement of the CFTR Scaffolding Protein NHERF1. Biomedicines. 2021 Jan 26;9(2):117.
Fabbri E, Tamanini A, Jakova T, Gasparello J, Manicardi A, Corradini R, Finotti A, Borgatti M, Lampronti I, Munari S, Dechecchi MC, Cabrini G, Gambari R. Treatment of human airway epithelial Calu-3 cells with a peptide-nucleic acid (PNA) targeting the microRNA miR-101-3p is associated with increased expression of the cystic fibrosis Transmembrane Conductance Regulator (CFTR) gene. Eur J Med Chem. 2021 Jan 1;209:112876. doi: 10.1016/j.ejmech.2020.112876
Gasparello J, Fabbri E, Gambari R, Finotti A. Differential effects on the miRNome of the treatment of human airway epithelial Calu-3 cells with peptide-nucleic acids (PNAs) targeting microRNAs miR-101-3p and miR-145-5p: Next generation sequencing datasets. Data Brief. 2021 Jan 9;35:106718. doi: 10.1016/j.dib.2021.106718. eCollection 2021 Apr.
Sultan S, Rozzi A, Gasparello J, Manicardi A, Corradini R, Papi C, Finotti A, Lampronti I, Reali E, Cabrini G, Gambari R, Borgatti M. A Peptide Nucleic Acid (PNA) Masking the miR-145-5p Binding Site of the 3’UTR of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) mRNA Enhances CFTR Expression in Calu-3 Cells. Molecules. 2020 Apr 5;25(7):1677. doi: 10.3390/molecules25071677
Finotti A, Gasparello J, Fabbri E, Tamanini A, Corradini R, Dechecchi MC, Cabrini G, Gambari R. Enhancing the Expression of CFTR Using Antisense Molecules against MicroRNA miR-145-5p.Am J Respir Crit Care Med. 2019 Jun 1;199(11):1443-1444. doi: 10.1164/rccm.201901-0019LE
Fabbri E, Tamanini A, Jakova T, Gasparello J, Manicardi A, Corradini R, Sabbioni G, Finotti A, Borgatti M, Lampronti I, Munari S, Dechecchi MC, Cabrini G, Gambari R. A Peptide Nucleic Acid against MicroRNA miR-145-5p Enhances the Expression of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) in Calu-3 Cells. Molecules. 2017 Dec 29;23(1):71. doi: 10.3390/molecules23010071
