Last generation 4,6,4′- trimethylangelicin (TMA) – derivatives with anti-inflammatory properties
update April 2023
Correction of the defective CFTR protein is the priority goal in the treatment of the lung pathology of patients affected by cystic fibrosis. The possibility that mutated CFTR correctors and potentiators currently in clinics are sufficient to intervene on the chronic inflammatory status of CF lungs is not yet confirmed. Innovative “CF tailored” anti-inflammatory drugs, to be applied as complementary treatments in parallel with CFTR correctors and potentiators is therefore an open issue. In this respect, the discovery of 4,6,4’-trimethylangelicin (TMA), an angular furocoumarin molecule with triple effects as F508del CFTR corrector and potentiator with additional anti-inflammatory action (Tamanini et al, 2011; Marzaro et al, 2013; Favia et al, 2014; Lampronti et al, 2017) has raised interest in the field (Collawn et al, 2014).
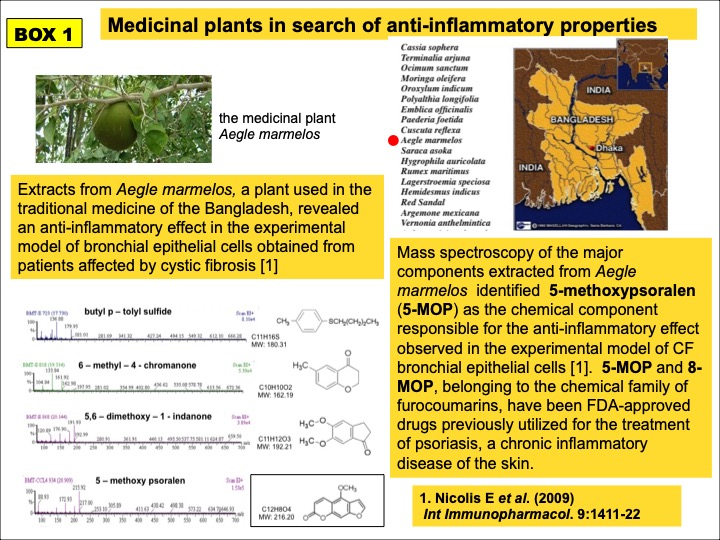
TMA discovery started from an initial screening of extracts from plants of the medicinal tradition of the Bangladesh, which led to the identification of the anti-inflammatory properties of the linear furocoumarin 5-methoxypsoralen (5-MOP) (BOX 1). 5-MOP analogues were subsequentely developed in a novel furocoumarin chemical library, leading to the angular psoralen TMA, further tested in different CF experimental models (BOX 2).
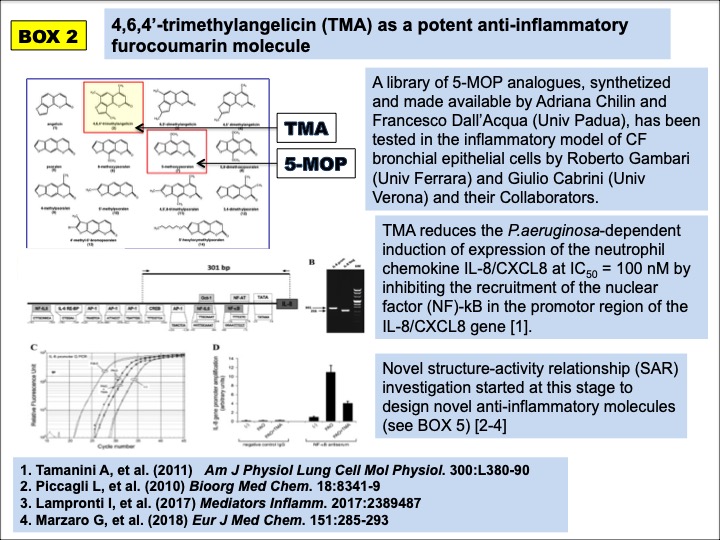
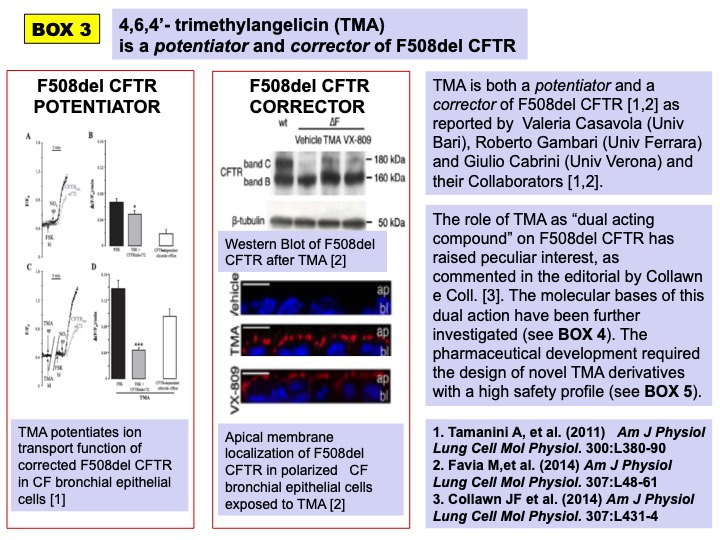
TMA was found to hinder the recruitment of the Nuclear Factor – kappaB (NFkB) on the promoter of the IL-8/CXCL8 gene in CF bronchial epithelial cells exposed to pro-inflammatory stimuli, thus reducing the expression of the main neutrophil chemokine, found abundantly released in the bronchial mucosa of CF patients. TMA was further tested for its properties on F508del CFTR. TMA at nanomolar concentrations was found to correct and potentiate F508del CFTR (BOX 3).
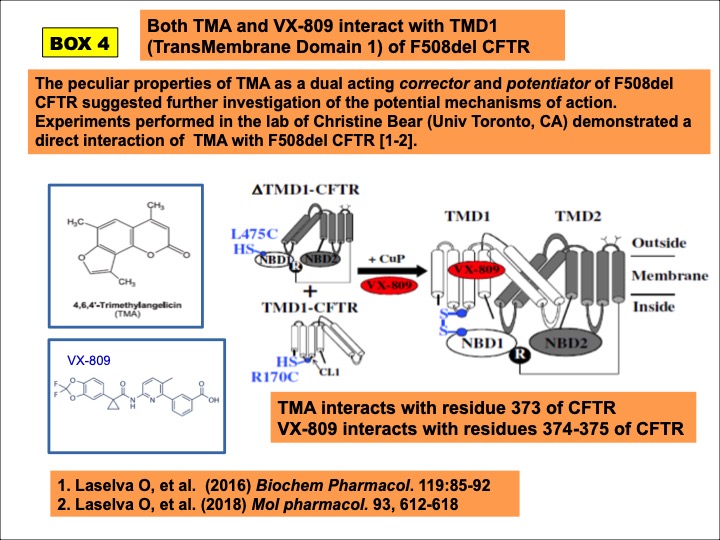
The dual acting activity of TMA as corrector and potentiator suggested further investigation on the potential interactions of TMA with F508del CFTR. Analyses in the lab of Christine Bear (Univ. Toronto, CA) showed that TMA interacts with residue 373 in the MSD1 (Membrane Spanning Domain 1) of CFTR stabilizing the interface MSD1 and CL4 (Laselva et al, 2016; Laselva et al, 2018a) (BOX 4).
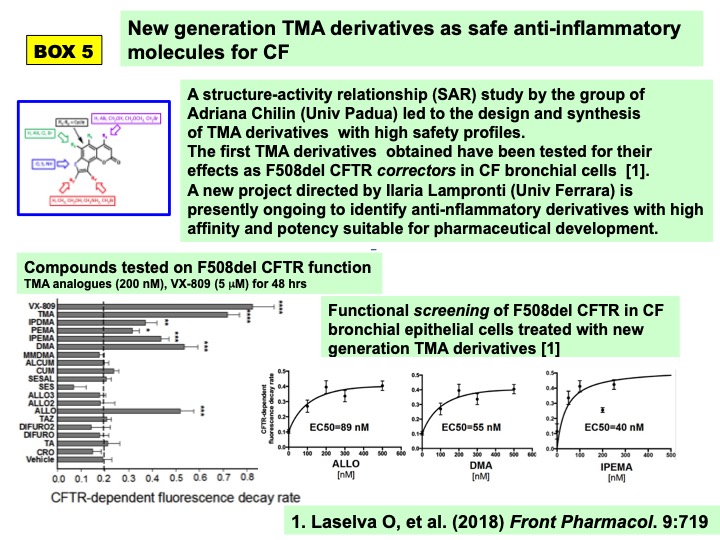
Industrial pharmaceutical development of 4,6,4′-trimethylangelicin for CF lung disease has been evaluated by Marco Prosdocimi and his colleagues Germano Carganico, Fabrizio Minoja and Anita Falezza (Rare Partners, Milan, IT), considering the steps of international patenting and designation as Orphan Drug for CF patients by the European Medicines Agency. This led to the indication of the need to design new 4,6,4′-trimethylangelicin analogues with high safety profile before proceding to further industrial development. As a result, Structure-Activity Relationaship (SAR) analyses of the group leaded by Adriana Chilin (University of Padua) allowed to design, synthetize and select TMA derivatives without any mutagenic and phototoxic effects (Vaccarin et al, 2022), setting novel formulations to improve bioavailability upon oral administration (Franceschinis et al. 2022). These novel TMA derivatives have been already studied as F508del CFTR modulators (Laselva et al, 2018b) and are presently investigated by the group leaded by Ilaria Lampronti as novel anti-inflammatory molecules for CF in an ongoing project financed by the Italian CF Research Foundation (BOX 5). Preliminary results led to identify the GY971a analogue as an interesting anti-inflammatory molecule acting mainly on IL-8 release, a CF hallmark of the excessive inflammatory process (Tupini et al. 2022).
References
Collawn JF, Fu L, Bartoszewski R, Matalon S. Rescuing ΔF508 CFTR with trimethylangelicin, a dual-acting corrector and potentiator. Am J Physiol Lung Cell Mol Physiol. 2014;307:L431-4.
Favia M, Mancini MT, Bezzerri V, Guerra L, Laselva O, Abbattiscianni AC, Debellis L, Reshkin SJ, Gambari R, Cabrini G, Casavola V. Trimethylangelicin promotes the functional rescue of mutant F508del CFTR protein in cystic fibrosi airway cells. Am J Physiol Lung Cell Mol Physiol. 2014;307:L48-61.
Franceschinis, E., Roverso, M., Gabbia, D., De Martin, S., Brusegan, M., Vaccarin, C., Bogialli, S., Chilin, A. (2022) Self-Emulsifying Formulations to Increase the Oral Bioavailability of 4,6,4′-Trimethylangelicin as a Possible Treatment for Cystic Fibrosis. Pharmaceutics 14, 1806
Lampronti I, Manzione MG, Sacchetti G, Ferrari D, Spisani S, Bezzerri V, Finotti A, Borgatti M, Dechecchi MC, Miolo G, Marzaro G, Cabrini G, Gambari R, Chilin A. Differential Effects of Angelicin Analogues on NF-kB Activity and IL-8 Gene Expression in Cystic Fibrosis IB3-1 Cells. Mediators Inflamm. 2017;2017:2389487.
Laselva O, Molinski S, Casavola V, Bear CE. The investigational Cystic Fibrosis drug Trimethylangelicin directly modulates CFTR by stabilizing the first membrane-spanning domain. Biochem Pharmacol. 2016;119:85-92.
Laselva O, Molinski S, Casavola V, Bear CE. Correctors of the Major Cystic Fibrosis Mutant Interact through Membrane-Spanning Domains. Mol Pharmacol. 2018a;93:612-618.
Laselva O, Marzaro G, Vaccarin C, Lampronti I, Tamanini A, Lippi G, Gambari R, Cabrini G, Bear CE, Chilin A, Dechecchi MC. Molecular Mechanism of Action of Trimethylangelicin Derivatives as CFTR Modulators. Front Pharmacol. 2018b;9:719.
Marzaro G, Guiotto A, Borgatti M, Finotti A, Gambari R, Breveglieri G, Chilin A. Psoralen derivatives as inhibitors of NF-κB/DNA interaction: synthesis, molecular modeling, 3D-QSAR, and biological evaluation. J Med Chem. 2013;56:1830-42.
Marzaro G, Lampronti I, D’Aversa E, Sacchetti G, Miolo G, Vaccarin C, Cabrini G, Dechecchi MC, Gambari R, Chilin A. Design, synthesis and biological evaluation of novel trimethylangelicin analogues targeting nuclear factor kB (NF-kB). Eur J Med Chem. 2018;151:285-293.
Nicolis E, Lampronti I, Dechecchi MC, Borgatti M, Tamanini A, Bezzerri V, Bianchi N, Mazzon M, Mancini I, Giri MG, Rizzotti P, Gambari R, Cabrini G. Modulation of expression of IL-8 gene in bronchial epithelial cells by 5-methoxypsoralen. Int Immunopharmacol. 2009;9:1411-22.
Piccagli L, Borgatti M, Nicolis E, Bianchi N, Mancini I, Lampronti I, Vevaldi D, Dall’Acqua F, Cabrini G, Gambari R. Virtual screening against nuclear factorκB (NF-κB) of a focus library: Identification of bioactive furocoumarin derivatives inhibiting NF-κB dependent biological functions involved in cystic fibrosis. Bioorg Med Chem. 2010;18:8341-9.
Tamanini A, Borgatti M, Finotti A, Piccagli L, Bezzerri V, Favia M, Guerra L, Lampronti I, Bianchi N, Dall’Acqua F, Vedaldi D, Salvador A, Fabbri E, Mancini I, Nicolis E, Casavola V, Cabrini G, Gambari R. Trimethylangelicin reduces IL-8 transcription and potentiates CFTR function. Am J Physiol Lung Cell Mol Physiol. 2011;300:L380-90.
Tupini, C., Chilin, A., Rossi, A., De Fino, I., Bragonzi, A., D’Aversa, E., Cosenza, L.C., Vaccarin, C., Sacchetti, G., Borgatti, M., Tamanini, A., Dechecchi, M.C., Sanvito, F., Gambari, R., Cabrini, G., Lampronti, I. (2022) New TMA (4,6,4′-Trimethyl angelicin) Analogues as Anti-Inflammatory Agents in the Treatment of Cystic Fibrosis Lung Disease. Int J Mol Sci. 23, 14483
Vaccarin, C., Gabbia, D., Franceschinis, E., De Martin, S., Roverso, M., Bogialli, S., Sacchetti, G., Tupini, C., Lampronti, I., Gambari, R., Cabrini, G., Dechecchi, M.C., Tamanini, A., Marzaro, G., Chilin, A. (2022) Improved Trimethylangelicin Analogs for Cystic Fibrosis: Design, Synthesis and Preliminary Screening. Int J Mol Sci. 23, 11528
